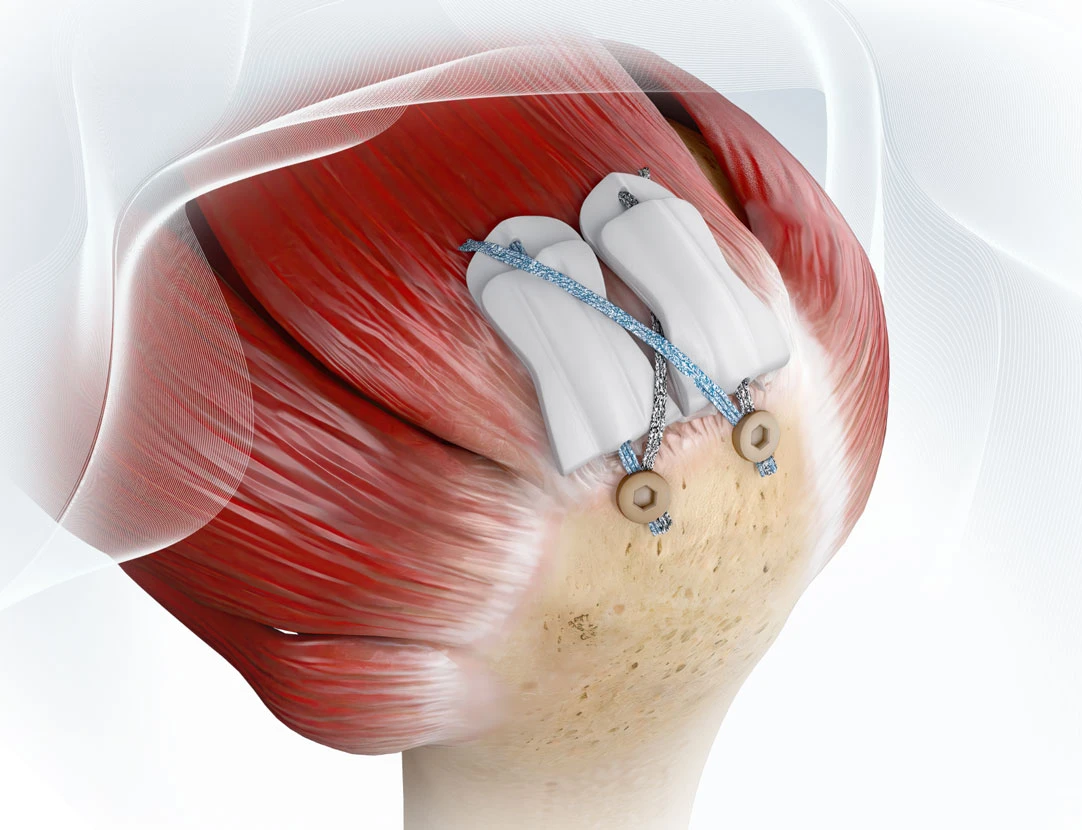
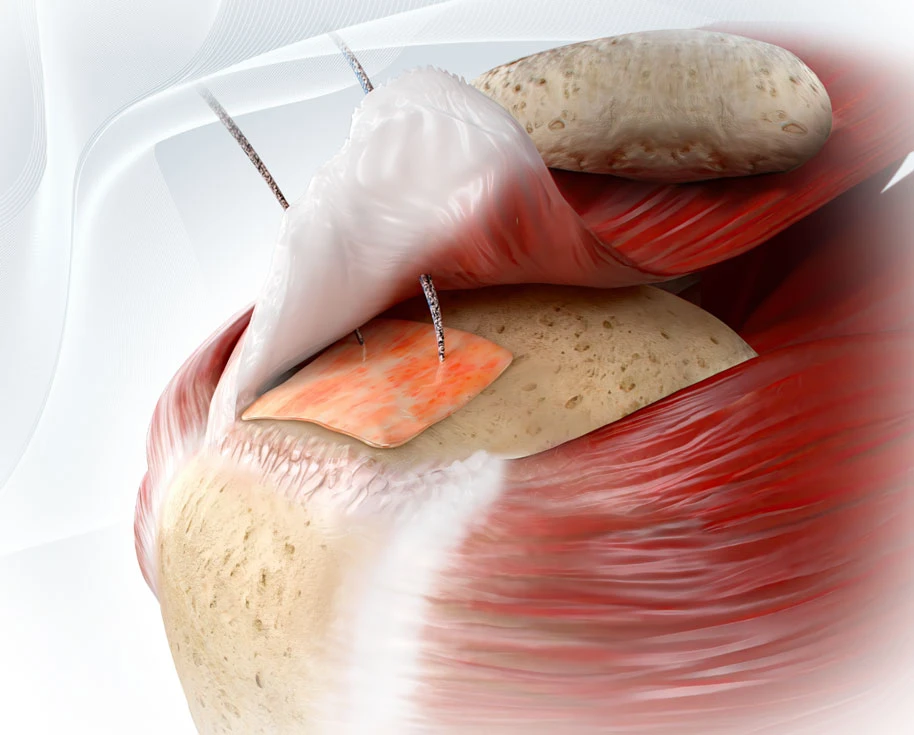
Rotator cuff repair (RCR) is one of the most commonly performed orthopedic procedures in the United States. While generally successful, structural failures still occur in up to 40% or more of patients, depending on tear size, tissue quality, and comorbidities. These failures can not only result in expensive downstream treatments, including repeat imaging, extended rehabilitation, and revision surgery, but also reduced functional improvements. In fact, patients with intact repairs tend to experience better functional outcomes and strength compared to those who suffer retears—outcomes that not only impact patient quality of life, but also influence downstream healthcare utilization and associated costs. Angerett et al., 2024
The Short-Term Economic Cost of Failure
A 2023 economic analysis estimated that failed RCRs performed in the U.S. in 2022 alone could generate over $438 million in downstream healthcare costs over two years. Of that, $229 million was attributed to nonoperative care following clinical failure, while revision surgeries added another $127 million in cost. Young et al., 2023
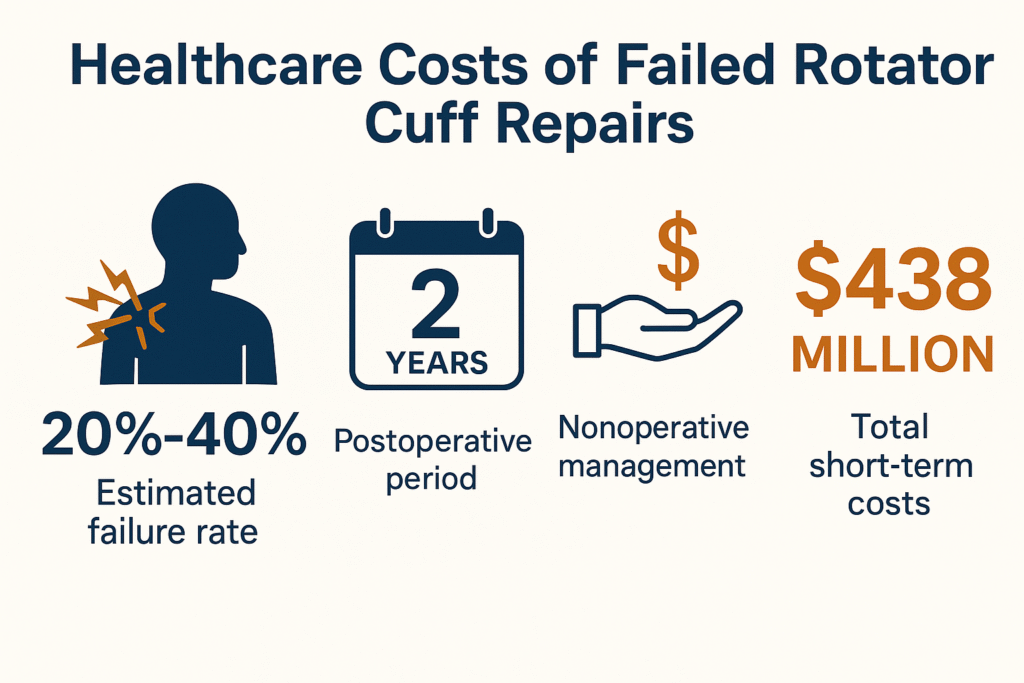
With failure rates hovering between 20% and 40% depending on patient and surgical factors, the need to improve biologic healing at the tendon-bone interface has become a clinical and economic imperative.
Why Biologic Augmentation Matters
Traditional approaches to RCR have focused on mechanical fixation. But many failures stem not from anchor pullout, but from poor biologic healing between tendon and bone. This is where biologic augmentation comes into play.
Technologies like the ROTIUM® Bioresorbable Wick and BioCharge® Autobiologic Matrix are designed to optimize the biologic environment where it matters most:
- ROTIUM® acts as an interpositional scaffold placed at the enthesis to support native tendon-bone integration. It retains the patient’s own biologic fluids at the interface, promoting scarless healing.
- BioCharge® targets the bursal side of the repair, improving healing at the tendon-suture interface and helping deter suture cut-through in compromised tissue.
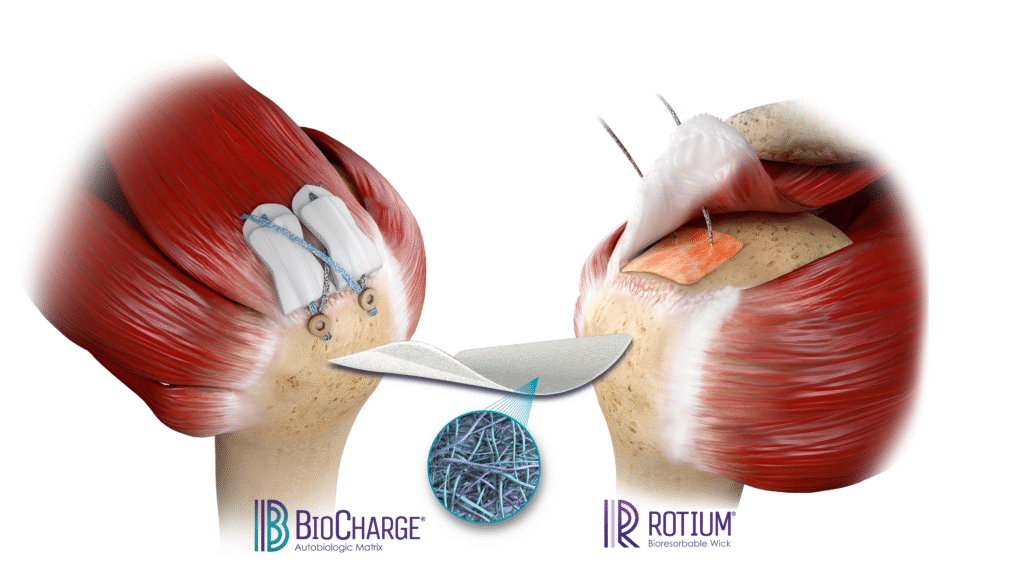
Both implants are fully synthetic, bioresorbable, and designed to integrate seamlessly into standard repair workflows without adding surgical time or requiring special instruments.
As Autobiologic implants, they work by concentrating, preserving, and promoting the patient’s own healing biology—rather than introducing donor tissue — and function as bioinductive scaffolds, encouraging native tissue remodeling at the repair site.
Clinical and Economic Implications
By enhancing the biologic healing cascade, these scaffolds may help reduce the likelihood of structural failure, which in turn could decrease the need for costly revisions. Cost-effectiveness analyses of similar biologic augmentations (e.g. ECM patches) show ICERs under $15,000/QALY, well below common willingness-to-pay thresholds.
Surgeons report the highest ROI when augmentation is used in large tears, revision cases, or biologically challenged patients (e.g. smokers, diabetics, older adults). In such cases, the biologic gap is wide—and the cost of failure is even higher.
Importantly, unlike many collagen-based patches, ROTIUM® and BioCharge® do not require additional instrumentation or costly disposable devices. Their design integrates directly into existing surgical workflows, minimizing added OR time, expense, and complexity. This makes them a more cost-effective option for biologic augmentation, especially in cost-sensitive settings like ASCs.
Conclusion
Reducing failure rates isn’t just good medicine, it’s good economics. As healthcare systems move toward value-based models, biologic augmentation offers a path to better outcomes at sustainable cost. And with technologies like ROTIUM® and BioCharge®, that path is already in reach.
Internal Links
References