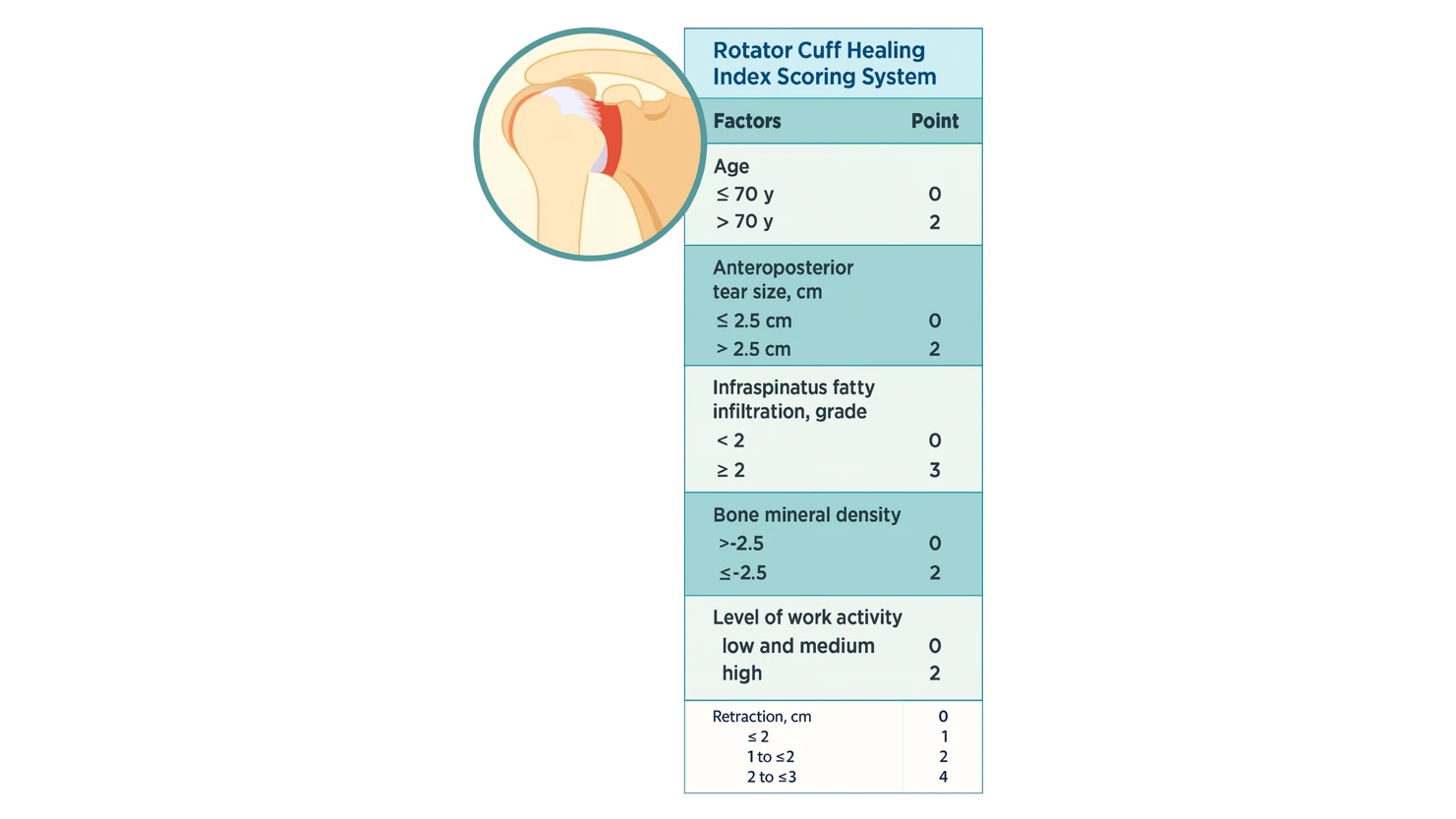
Rotator Cuff Healing Index (RoHI): Predicting Healing Success After Repair
Understanding the Rotator Cuff Healing Index (RoHI) Rotator cuff repair

Understanding the Rotator Cuff Healing Index (RoHI) Rotator cuff repair
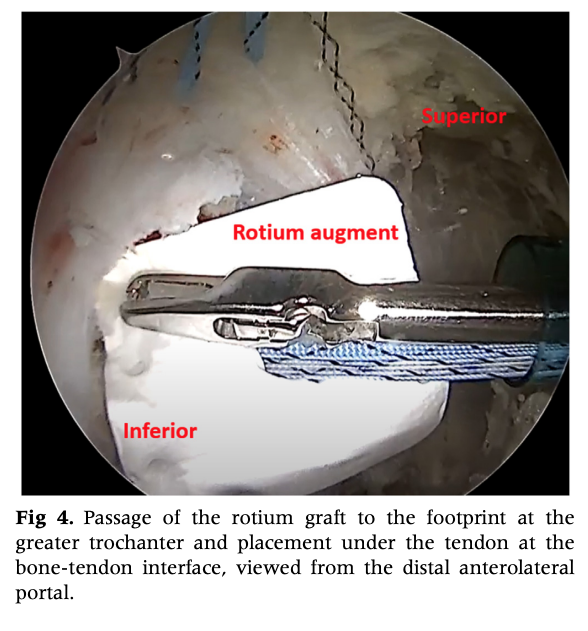
A New Era in Hip Tendon Repair: Biologic Augmentation with
BoneZone’s coverage of scaffold augmentation in rotator cuff surgery highlights key findings from a study that evaluated ROTIUM® Bioresorbable Wick, Atreon’s synthetic tendon-to-bone scaffold.

Atreon Orthopedics has received FDA 510(k) clearance to expand the indications for its ROTIUM® Bioresorbable Wick, now approved for use in tendon repair surgeries. This clearance allows the ROTIUM implant to support healing in various tendon injuries, broadening its application beyond rotator cuff repairs.

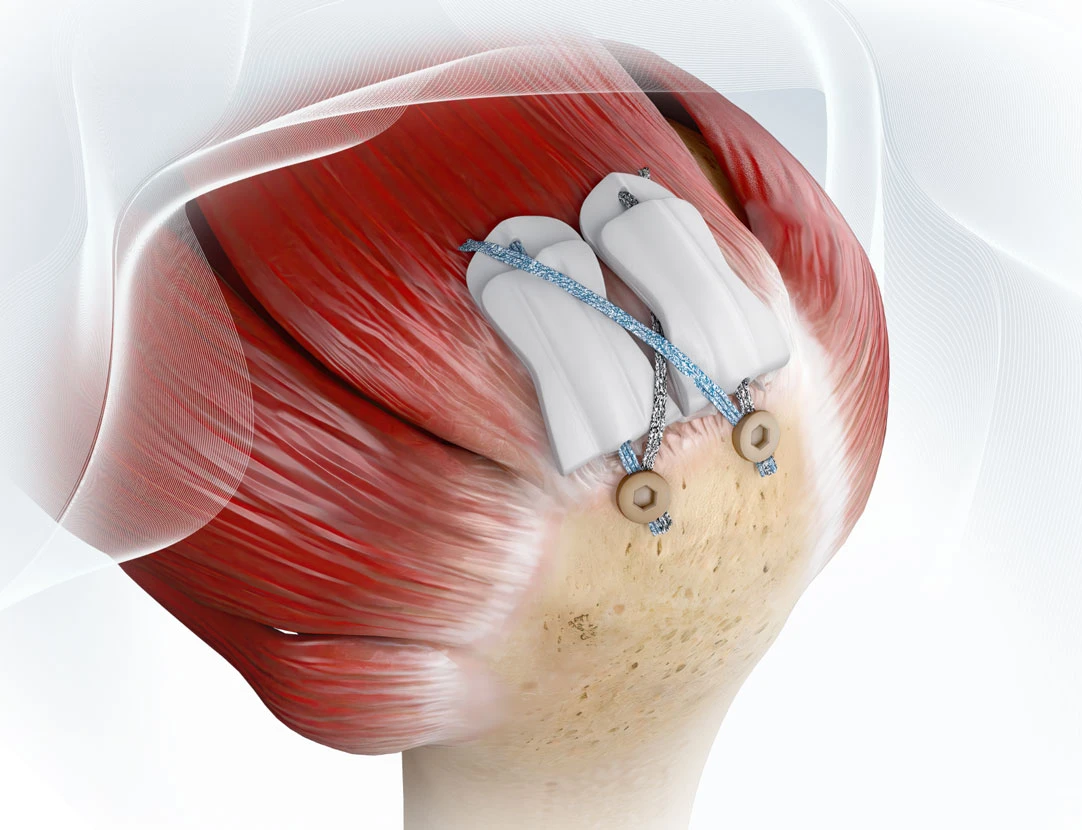
Atreon Orthopedics, LLC announces the FDA 510(k) Clearance and full market launch of BioCharge® Autobiologic Matrix, a bioresorbable synthetic implant.
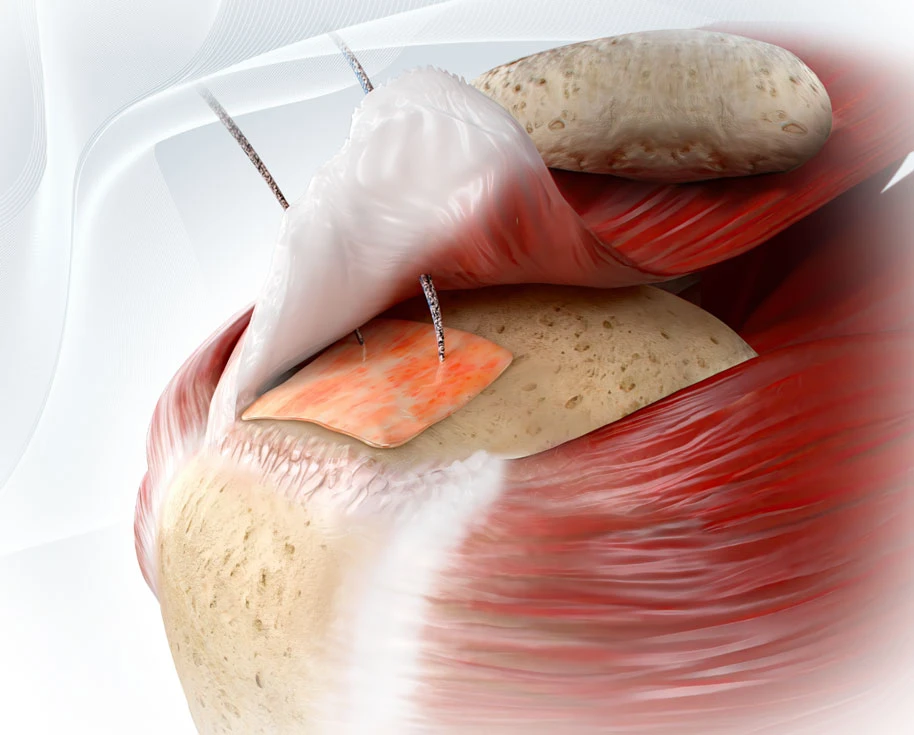
Atreon Orthopedics is proud to announce the successful use of its ROTIUM® Bioresorbable Wick in over 10,000 rotator cuff repair surgeries.
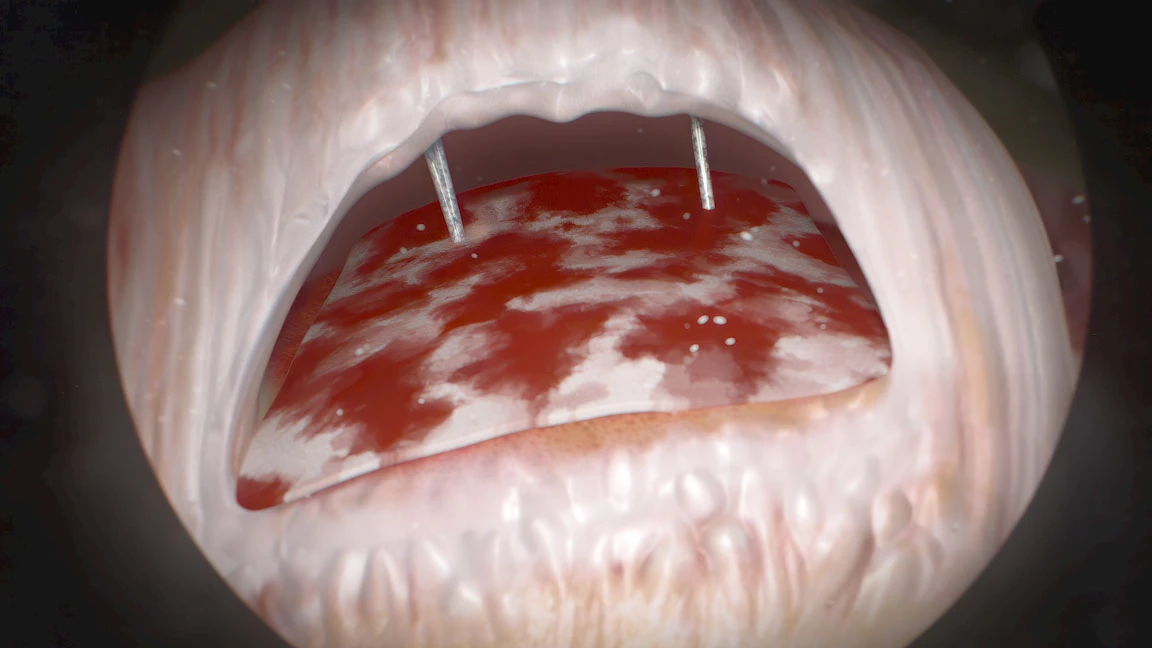
Short-term radiographic and clinical outcomes of arthroscopic rotator cuff repair with and without augmentation with an interpositional nanofiber scaffold. Journal of Orthopaedic Experience & innovation.
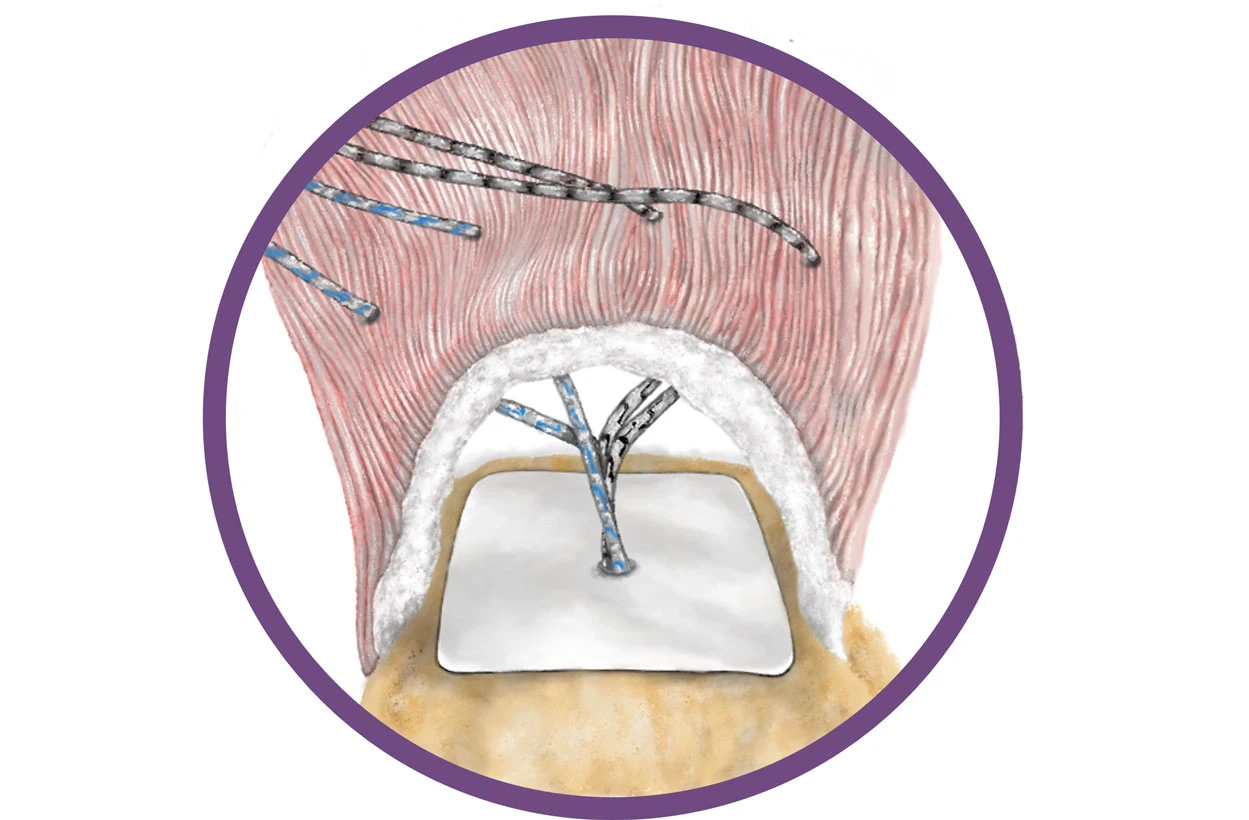
Beleckas, C. M., Bishai, S.K., & Badman, B. L. (2023). Rotator Cuff Repair Augmented with Interpositional Nanofiber Scaffold. Arthroscopy Techniques.
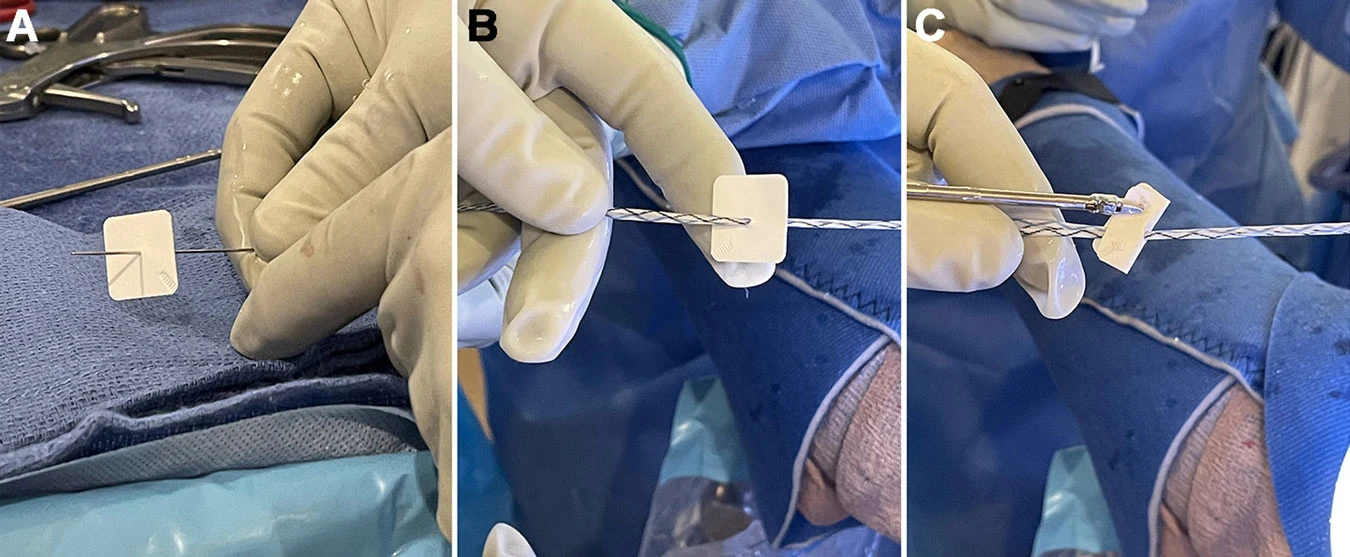
Seetharam A, Abad J, Baessler A, Badman BL. Use of a Nanofiber Resorbable Scaffold During Rotator Cuff Repair: Surgical Technique and Results After Repair of Small- to Medium-Sized Tears. Orthop J Sports Med. 2022 May 13;10(5).

Animal Studies: Romeo, A., Easley, J., Regan, D., Hackett, E., Johnson, J., Johnson, J., Puttlitz, C., & McGilvray, K. (2022). Rotator cuff repair using a bioresorbable nanofiber interposition scaffold: A biomechanical and histologic analysis in sheep. Journal of Shoulder and Elbow Surgery, 31(2), 402–412.